

The safety profile of Opdivo plus Yervoy and two cycles of chemotherapy was reflective of the known safety profiles of the immunotherapy and chemotherapy components in first-line NSCLC. The CHMP adopted the positive opinion based on results from the Phase 3 CheckMate -9LA trial, which met the primary endpoint of superior overall survival (OS). “We look forward to the EC’s decision and hope to soon introduce this innovative, dual immunotherapy approach to patients across the EU who may benefit.”
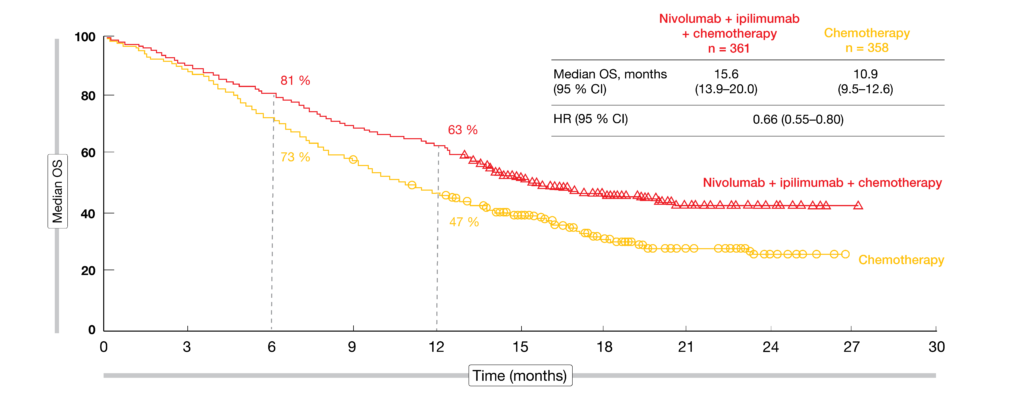
“This positive CHMP opinion reflects the potential for Opdivo plus Yervoy with two cycles of chemotherapy to offer the chance for a longer life to patients across subgroups of metastatic NSCLC, a devastating cancer where unmet needs still exist,” said Abderrahim Oukessou, M.D., vice president, thoracic cancers development lead, Bristol Myers Squibb. The European Commission (EC), which has the authority to approve medicines for the European Union (EU), will now review the CHMP recommendation. chemotherapy across all PD-L1 expression levels and histologiesīristol Myers Squibb (NYSE: BMY) today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended approval of Opdivo ® (nivolumab) plus Yervoy ® (ipilimumab) with two cycles of platinum-based chemotherapy for the first-line treatment of metastatic non-small cell lung cancer (NSCLC) in adults whose tumors have no sensitizing EGFR mutation or ALK translocation. Application based on results from the Phase 3 CheckMate -9LA trial, which showed significantly improved overall survival in previously untreated patients with metastatic non-small cell lung cancerĬlinical benefit with Opdivo plus Yervoy with limited chemotherapy observed vs.


 0 kommentar(er)
0 kommentar(er)
